Just like us, batteries follow their natural attractions — not to earn success or love, but to create chemical reactions. These interactions, when scaled up from a battery cell to the pack level, produce intense power – enough to deliver hundreds of miles of range across our EV portfolio.
If you’ve heard anything about EV batteries, you’ve probably heard the term “lithium-ion.” Today, lithium-ion batteries are the standard for most EVs. And you don’t need to be a master chemist to understand how these batteries work.
The process can be boiled down to something as simple as a game of tag. In this game of tag, the objective is simple: lithium ions and electrons (our players in this game) are trying to reunite. As they run after each other, they generate energy to power your vehicle.
Game Setup:
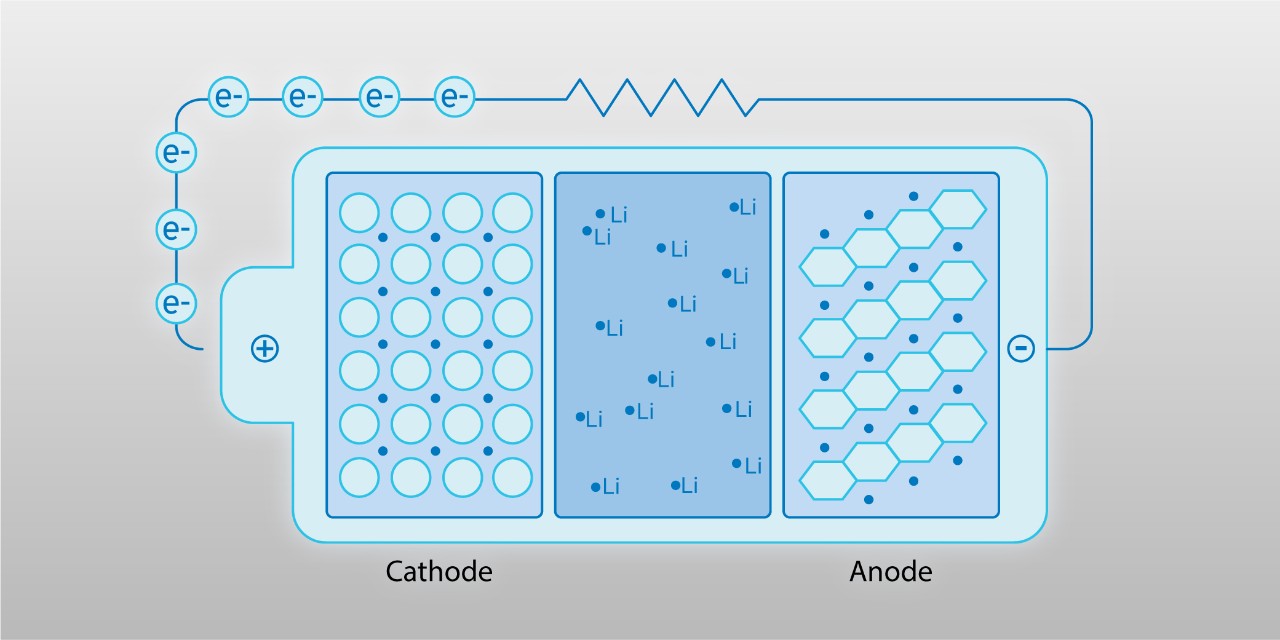
When you peel open a battery cell, you’ll see something that looks like a stack of papers. These are called electrodes. If we zoom into a few of these layers, you’ll witness how the game is set up – like two fields of play with a fence dividing them. The key sections are:
- The Anode – the starting playing field for lithium ions and electrons
- The Cathode – the destination playing field
- The Separator – a fence that the electrons can’t naturally cross
- The Terminals – the gates that the electrons use to leave and re-enter the cell
Before the game begins (when your EV is fully charged), all the particles wait at the anode, getting ready to play. Without getting too deep into the physics, the electrons and lithium ions that start in the anode of a battery cell spontaneously want to move to the cathode because that will lower the potential energy – and nature favors moving toward lower energy (much like gravity naturally causes objects to move downhill).
Rules of the Game: Drive Mode
- You turn on your car, signaling the start of the game
- The negatively charged electrons start moving to the positively charged cathode. Remember, they can’t move through the separator (our fence), so they travel outside the cell through the car’s wiring using the terminals (our gate).
- As electrons race through the wiring, they create electricity—lighting up your dashboard, spinning the wheels, and keeping everything powered.
- As the electrons move to the cathode, the positively-charged lithium ions work to try and catch up – balancing the electrons’ negative charge. However, the lithium ions have a much easier route; they are naturally able to just slip through the separator. Think of a crafty player in this game of tag finding a way to sneak through the fence.
- Electrons and lithium ions have ended up in the cathode, where they come together in a process called intercalation, and the game is over.
Next Level: Recharge Mode
Once all the players reach the cathode, your battery is out of usable energy and the players rest together. But unlike your old AA batteries, EV batteries are rechargeable, getting you right back to square one.
Here’s how it works:
- You plug in your EV. This activates our electron and lithium ion players.
- Electricity from the charger pushes the electrons back out of the terminal and through their outside route – this time moving from the cathode to the anode.
- The lithium ions follow through the separator, following the electrons again to balance their negative charge with a positive one.
- Everyone reunites at the anode and rests up for the next round.
That power supply from the charger is key here, as the electrons and lithium ions don’t naturally want to move from cathode to anode. It’s like providing the players in our game with the boost they need to get them back to the anode starting point, where they’re ready to jump back into action the next time you start your EV.
This game of tag is what makes electric vehicles possible. It’s all about the desire of electrons and lithium ions to stick together.
So the next time you start your car, turn on your air conditioning, or listen to the stereo, remember you're not just driving—you're hosting one of the world’s smallest and fastest games of tag.
Check out more from the Plugged In series: